Magnetoencephalography (MEG) is an advanced, noninvasive neurophysiology imaging technique, with wide ranging applications in the fields of neuroscience and medicine. MEG measures extracranial magnetic fields generated by the electrical activity in the brain (primarily the intracellular current flow in the pyramidal neurons), and provides a direct measure of brain function. MEG sensors record mixtures of electromagnetic signals emanated from different areas across the entire brain, and non-brain noise. To measure the minute magnetic fields produced by the brain (of the order of femto to pico Tesla), MEG uses superconducting quantum interference device (SQUID) sensors, which are immersed in liquid helium to cool them down to approximately -270 °C; the MEG system itself is usually housed in a magnetically shielded room (figure 1).
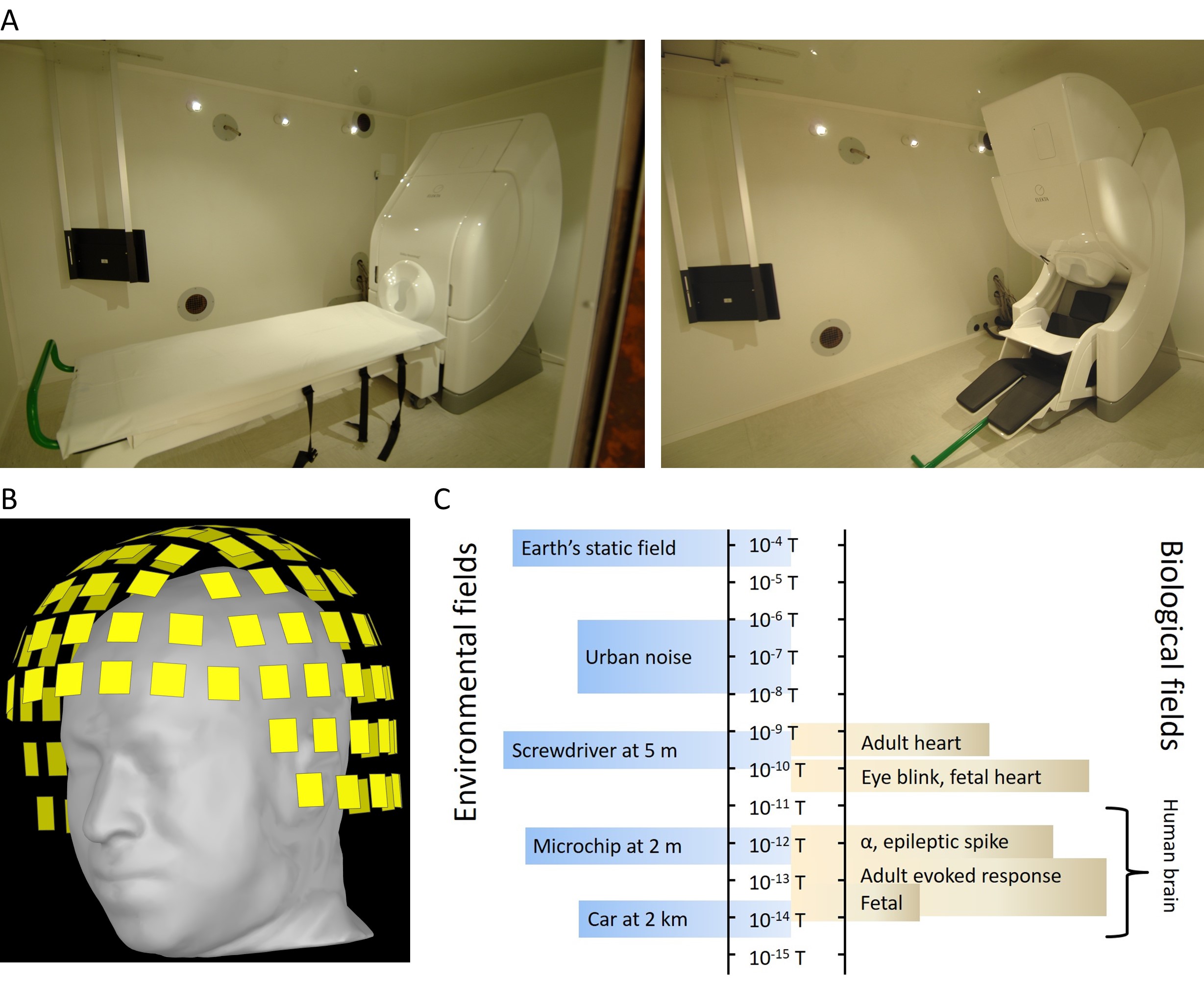
Figure 1. A) Photographs of the MEG system (306-sensor whole-head Vectorview system, Elekta Oy, Helsinki, Finland) inside a magnetically shielded room at the King Fahad Medical City in Riyadh, in supine (on the left) and seated (on the right) positions. B) Schematic image of 306 MEG sensors covering the head. C) Magnetic field strengths from different environmental and biological sources.
These signals are typically “cleaned” offline using various signal processing techniques, with an aim to keep only the brain signals of interest. Magnetic source imaging (MSI, e.g. dipole fit or distributed source analysis) is then used to localize neural sources of “clean” signals and map them on structural MRI (figure 2).
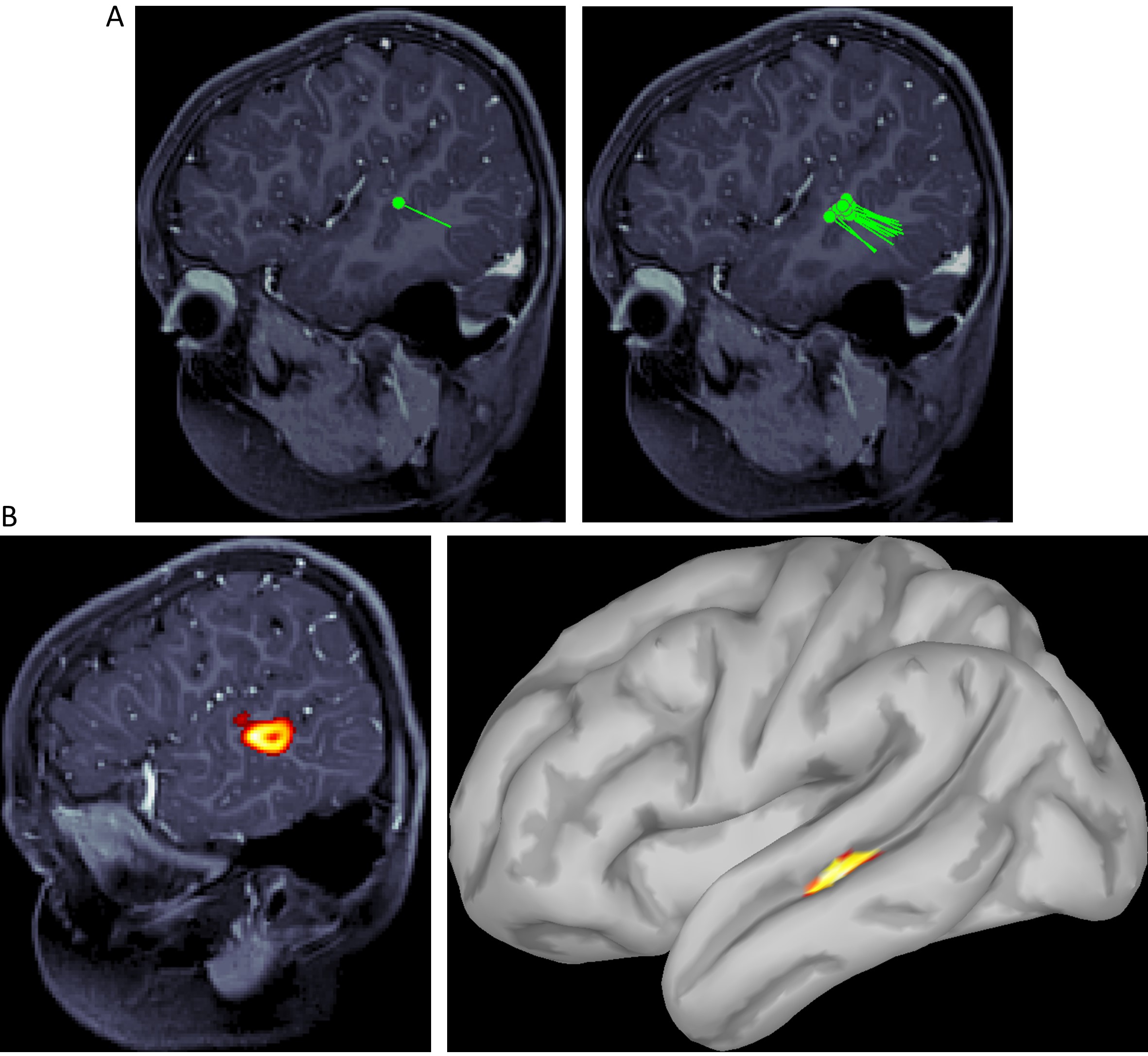
Figure 2. Examples of various MSI or MEG functional localization maps. A) Equivalent current dipole (ECD) fit. A single dipole (on the left) and a dipole cluster (on the right) are shown projected on the structural MRI. B) Distributed source activity is shown projected on the structural MRI (on the left) and cortical surface (on the right).
The unique value of MEG in research and clinical applications stems from its noninvasiveness, high spatial and temporal resolution (of the order of few millimeters and submillisecond respectively), and the ability to provide direct access to the electrophysiological activity throughout the entire brain. MEG is able to track neuronal activity at its characteristic time scale (i.e., milliseconds), and is well-suited for studying the localized as well as large-scale dynamics of brain activity, and interregional functional connectivity. This characteristic allows MEG also to investigation, with high spectral resolution, neural oscillations, which are known to play important roles in cognitive processes.
CLINICAL APPLICATIONS
The current principal clinical use of MEG is in the fields of epilepsy and neurosurgery; specifically, it is routinely used to estimate epileptogenic zone and map functionally eloquent cortex. There is strong evidence in the literature supporting the use of MEG in presurgical evaluation of patients with drug-resistant epilepsy, especially in determining good candidates for epilepsy surgery and intracranial EEG study, guiding the placement of invasive electrodes to improve the yield of intracranial EEG, and estimating the location and extent of the epileptogenic zone to aid in determining the resection margins (figure 3).
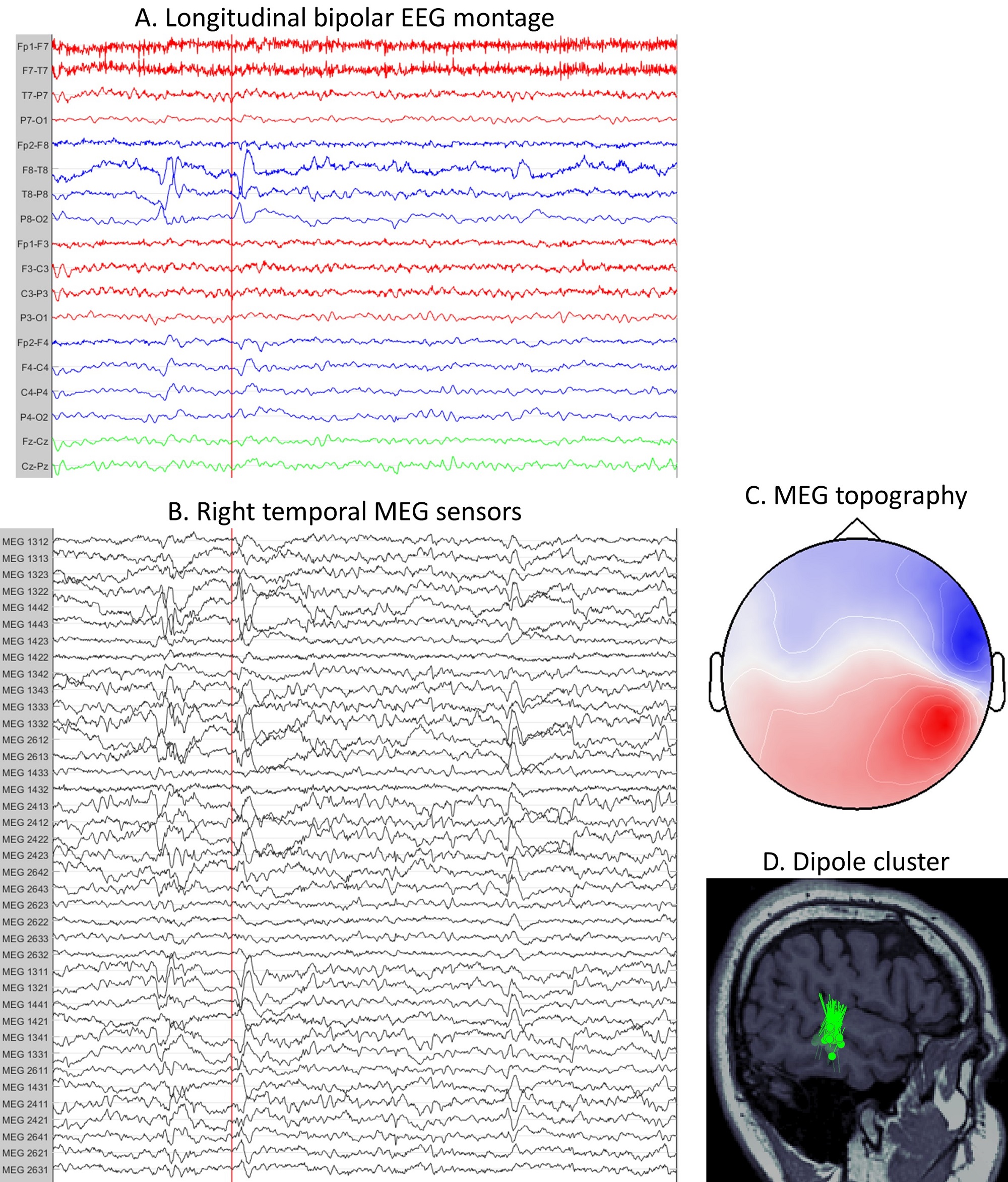
Figure 3. Localization of epileptic focus from MEG data. EEG signals are recorded simultaneously and in synchrony with MEG. A) EEG signals in a standard longitudinal bipolar montage (“double banana”). A 10 s window with epileptic spikes is shown. The red line is placed near the onset of the spike. B) MEG signals from only the right temporal sensors. The same 10 s window as in figure A is shown. The spike can be seen on both MEG (figure B) and EEG (figure A) data. C) Topography of MEG signals at the peak of the spike. A nice dipolar pattern can be seen over the right temporal sensors. D) MEG dipole cluster showing the location of the irritative zone, as a surrogate for the epileptogenic zone. Each dipole is obtained by localizing a single interictal spike at its peak. The tight cluster of dipoles indicates that all spikes are generated in the same brain region. Surgical resection of such clusters typically leads to seizure freedom in around 90% of patients.
Clinical value of MEG in other areas of epilepsy management and surgery is less established. However, some research results suggest that MEG may facilitate the study of various aspects of the disease and be used in the diagnosis and characterization of epilepsy types, in the monitoring of treatment response and predicting treatment outcome.
Regarding the mapping of functionally eloquent cortex, there is strong research and clinical evidence supporting the use of MEG for the assessment of the hemispheric dominance of language function and localization of primary sensorimotor cortex. These procedures are in routine clinical use in many epilepsy centers. The evidence for the MEG-based localization of receptive and expressive language functions, while extant, is not as strong, and is only seldom used in clinical practice to provide supporting information. Currently, only few MEG studies have been conducted on presurgical mapping of memory function, providing a preliminary evidence on its clinical utility. Functional mapping of visual and auditory cortex is used mostly in basic research studies and is of limited clinical value.
RESEARCH APPLICATIONS
In research, MEG has been used to study all aspects of normal and pathological brain functions, revealing large-scale brain dynamics and neurofunctional mechanisms underlying variety of normal mental processes as well as dysfunctions in neurological and psychiatric diseases. Innovative and unique results have been obtained in topics of systems and cognitive neuroscience, such as vision, audition, sensorimotor, memory, attention, language and others. In clinical research, most significant findings have been obtained in studies of epilepsy, schizophrenia, autism spectrum disorders, Alzheimer’s disease, post-traumatic stress disorder, stroke, Parkinson’s disease and others.
Author:

Dr Vahe Poghosyan
Head – MEG Unit
Department of Neurophysiology, National Neurosciences Institute,
KIng Fahad Medical City, Riyadh, K.S.A

